
During the COVID-19 pandemic, many chemical and consumer goods companies have announced that they have retooled to make hand sanitizer for use by consumers and medical professionals. Also, many chemical firms have showcased hand sanitizer formulas using their proprietary ingredients. However, potential manufacturers should keep in mind that the formulation of hand sanitizers is regulated in the United States by the Food & Drug Administration (FDA) and the regulations can be confusing.[1]
The FDA considers medical and consumer hand sanitizers over the counter (OTC) drugs.[2] There are regulations on hand sanitizers that govern their composition, manufacturing conditions, and what product claims can be made. Deviating from those regulations puts manufacturers at risk of FDA enforcement actions such as a warning letter or a product recall notice. In January 2020, Purell® maker GOJO Industries received an FDA warning letter concerning its claims language, not its formulation.[3] It’s acceptable to claim (with evidence) that hand sanitizers reduce the level of bacteria on the skin, but not that they kill disease-causing bacteria and viruses, because evidence for that claim is nonexistent.
The FDA has issued very specific guidance on hand sanitizers.[4] There are two kinds of hand sanitizer: liquid and gelled. Manufacturing of liquid hand sanitizers is temporarily allowed during the COVID-19 health emergency, but some requirements must be followed, including that they can only contain USP grade denatured alcohol, glycerin, hydrogen peroxide, and sterile distilled water. The requirements that the manufacturer must use Good Manufacturing Principles (GMP), must follow labeling rules, and that new drug compositions require a New Drug Application (NDA), are temporarily waived.
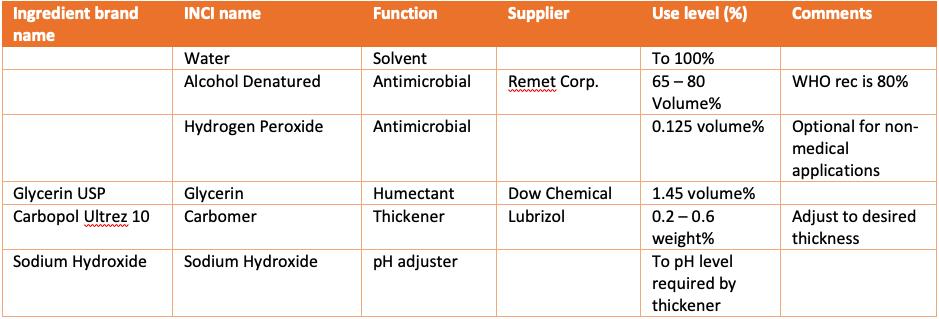
The FDA recommended formulation for hand sanitizers conforms to the formula recommended by the World Health Organization, which has 80 vol% ethanol or 75 vol% isopropyl alcohol in aqueous solution, combined with 1.45 vol% glycerin, 0.125 vol% hydrogen peroxide, and distilled water, with no other ingredients. The hydrogen peroxide is present due to possible contamination of the other ingredients by bacterial spores and may be omitted if proper manufacturing cleanliness is observed.
The other kind of hand sanitizer is a gelled formula referred to in the FDA monograph as an instant hand sanitizer. Non-active ingredients in OTC instant hand sanitizers must be USP or NF grade excipients according to the monograph. Important for gelled hand sanitizers is that the only USP-grade thickeners are carbomer, clays (e.g. attapulgite, bentonite, kaolin), and polysaccharides (e.g. xanthan gum, hydroxyethyl cellulose).[5] Adding a non-USP grade thickening polymer may make the FDA consider the product “misbranded”, meaning misleadingly labeled. The likelihood of the FDA taking action in the current environment may be low, however, especially since carbomer has been in short supply.
Gel hand sanitizing products with a non-USP thickener should not make claims about being antiseptic and reducing bacteria and viruses. Only products that follow the formulas in the FDA guidance document can claim to be hand sanitizers. We recommend a formulation for a hand sanitizing gel based on alcohol, water, glycerin, and a thickening polymer with a pH neutralizer. The formula should meet FDA guidelines for a hand sanitizer other than that the thickener is not USP grade.
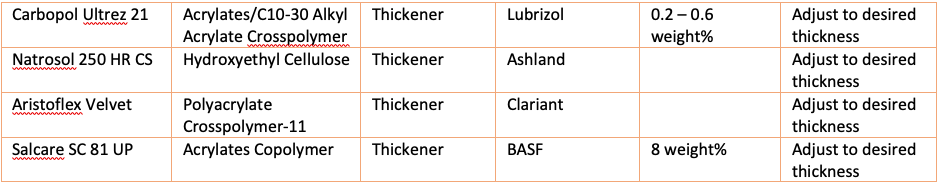
To make the formula into a gel, a thickening polymer can be used. Generally, carbomer is recommended, but this has been in short supply during the COVID-19 crisis. Sanitizing efficacy should not be affected if other thickeners are used. However, as stated products with non-USP grade thickeners cannot make claims about their antiseptic efficacy, even if supporting data is available.
Recommended formulas:
Other possible thickeners that can be tried:
It is also possible to add emollients and an emulsifier for a creamier formula, which may not be as effective. All these materials can be obtained from a chemical distributor such as Azelis, Brenntag, Univar, and others.
Caution: alcohol is highly flammable and proper precautions should be used when handling this material. Please refer to the Safety Data Sheet.
[1] Rachel Grabenhofer, David Steinberg, “How NOT to Formulate Hand Sanitizers”, Cosmetics & Toiletries April 23, 2020.
[2] Matthew Zoeller, “U.S. FDA Hand Sanitizer Solutions Explained”, Cosmetics & Toiletries May 1, 2020.
[3] https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/gojo-industries-inc-599132-01172020 Retrieved May 26, 2020.
[4] U.S. FDA Guidance Document “Policy for Temporary Compounding of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency”, FDA-2020-D-1106 (March 2020).
[5] Excerpted USP-NF and FCC Standards: A Hand Sanitizer Resource; A collection of standards provided as a resource to assist with the challenges posed by COVID-19, https://www.usp.org/sites/default/files/usp/document/health-quality-safety/usp-hand-sanitizer-ingredients.pdf Retrieved May 26, 2020.
